JAK INHIBITORS AND JAK2 MUTATION: WHAT’S THE CONNECTION?
February 6, 2014
From MPN Focus, a newsletter from the Clinical Research Center for MPNs at MD Anderson Cancer Center
By Kate Newberry & Srdan Verstovsek
The JAK2V617F mutation is the most prevalent gene mutation among patients with myeloproliferative neoplasms (MPNs), and its discovery led to the development of JAK inhibitors, which have transformed the treatment landscape for myelofibrosis (MF). While 95% of patients with polycythemia vera (PV) harbor the JAK2V617F mutation, only 50-60% of patients with essential thrombocythemia (ET) and MF have the mutation. More importantly, results from clinical trials of ruxolitinib (Jakafi) and other JAK inhibitors, show that patients have significant improvements in symptoms and quality of life regardless of whether they have this mutation or not. So, what exactly is the connection between the JAKV617F mutation and JAK inhibitors?
First a bit about the role of JAK signaling in MPNs:
Under normal conditions, JAKs (short for Janus kinases; there are 4 members of the JAK family of proteins: JAK1, JAK2, JAK3, and TYK2) play important roles in the development of blood cells and regulation of the immune system. JAKs are molecules inside the cell that interact with structures called receptors that are present on the surface of blood and immune system cells. Receptors protrude through the cell membrane. On the outside of the cell they are a docking site for different proteins called cytokines and growth factors. When cytokines (e.g., interleukins and interferons) or growth factors (e.g., erythropoietin [EPO], a growth factor for red blood cells, and thrombopoietin [TPO], a growth factor for platelets) bind to the receptors, the JAK molecules are activated. Once activated, STAT (signal transducers and activators of transcription) molecules are recruited to the receptor, where they can be activated by JAKs. STAT molecules then travel to the nucleus (a structure in the center of the cell containing chromosomes that carry genes), where they are responsible for turning on genes (transcription) involved in many basic cellular functions, including cell growth and survival (Figure 1).
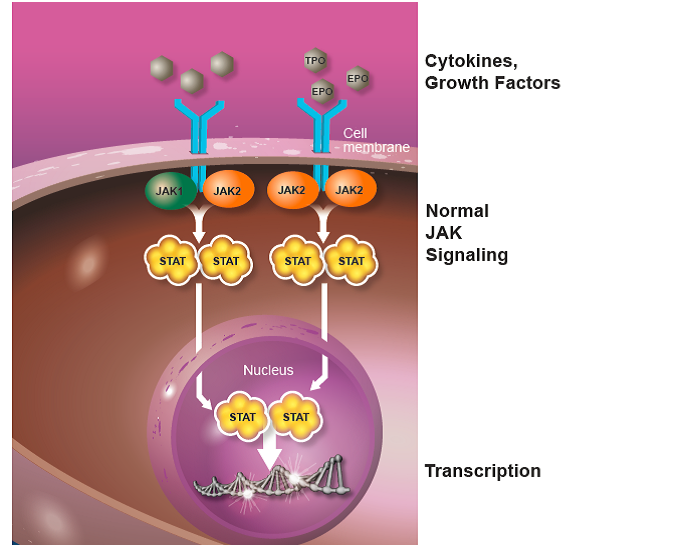
Figure 1. The JAK-STAT signaling pathway. Normal JAK-STAT signaling is mediated by cytokines and growth factors, including erythropoietin (EPO) and thrombopoietin (TPO), which are essential for normal blood cell formation. The receptors are show in blue and the cytokines/growth factors in grey.
Unchecked activation of the JAK-STAT pathway results in uncontrolled cell growth and survival, which can lead to pathological states such as MPN (Figure 2). This increased activation (dysregulation) of the JAK-STAT pathway can occur through activating mutations in the JAK2 gene (i.e., JAK2V617F), exaggerated expression of cytokines that activate JAK proteins, or through other mutations that disrupt the function of molecules that turn off the JAK-STAT pathway. Increasing evidence suggests that dysregulated JAK-STAT signaling plays a central role in the development of MPNs, but there are likely mutations in other genes, which have not yet been discovered, that are also contributing to the development of MPNs.
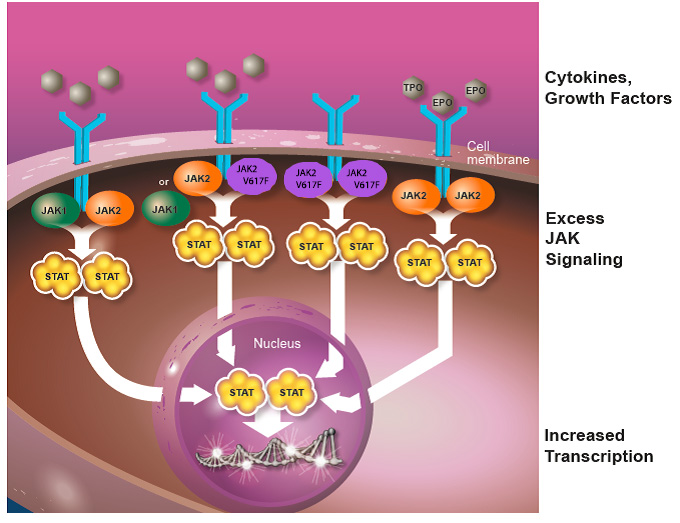
Figure 2. Dysregulated JAK-STAT signaling. Increased levels of cytokines and the JAK2V617F mutation lead to persistent activation of the JAK-STAT pathway. Increased JAK signaling results in a pro-inflammatory state caused by overproduction of cytokines, which is thought to cause many of the symptoms experienced by patients with MF, including fatigue, night sweats, itching and bone pain.
How do JAK inhibitors work?
Clinical studies of ruxolitinib and other JAK inhibitors have shown that they significantly improve many of the worst symptoms of MF, such as fatigue, night sweats, itching and bone pain, reduce splenomegaly, and improve quality of life. Importantly, these beneficial effects are seen regardless of whether the patient harbors the JAK2V617F mutation. This is thought to be caused by the inhibitory effects of ruxolitinib on JAK proteins, specifically JAK1 and JAK2, which reduces the production of pro-inflammatory cytokines and controls cell growth and survival. While the JAK2V617F mutation results in constitutive activation of the JAK-STAT pathway, it is currently understood that in the addition to the uncontrolled cell growth and survival, an exaggerated production of cytokines mediated by JAK proteins is another important feature of MF that is thought to cause many of the associated symptoms, including fever, night sweats, pruritus, and weight loss. In fact, studies have shown an association between reductions in cytokine levels in patients on ruxolitinib therapy and improvements in their symptoms.
Is testing for the presence of the JAK2V617F mutation necessary?
While the discovery of the JAK2V617F mutation was an important turning point in the treatment of MF as well as our understanding of MPNs, its presence is not important for deciding whether a patient will derive benefit from a JAK inhibitor. However, testing for the mutation can help physicians make a proper diagnosis, which is critical for determining the optimal treatment plan. For example, since 95% of patients with PV have the mutation, and its presence along with an increased red blood cell mass (erythrocytosis) or abnormally high hemoglobin and low EPO is highly suggestive of PV. For those with ET and MF where the JAK2 mutation is only present in 50-60% of patients, the JAK2V617F mutation is not a major diagnostic characteristic. For ET and MF, JAK2 mutation testing is only another piece of the puzzle that still needs to be associated with clinical, biologic, and pathologic data to allow proper diagnosis.
Much has been learned about the molecular biology of MPNs since the discovery of the JAK2V617F mutation in 2005, but the precise causative factor remains an enigma. Current and future studies are aimed at unraveling the complexity of these neoplasms and identifying new targets for therapy. However, the take-home message is that JAK inhibitors are a promising treatment option for all patients with symptomatic MF, either those with symptomatic splenomegaly or with MF-related systemic symptoms regardless of whether they have the JAK2V617F mutation.
